 Introduction
Introduction
As antibiotic resistance continues to outpace our current drugs, scientists are exploring innovative solutions. In the study published in in Cell Biomaterials, our team have unveiled a novel approach to tackling antibiotic-resistant bacteria. By designing synthetic peptides that attack pathogens on two fronts, the work has opened new possibilities in the fight against superbugs.
A Dual-Action Strategy Against Resistant Bacteria
The peptides were uniquely designed to achieve what few treatments can—directly destroying bacteria while also stimulating the body’s immune defenses, working through three key features:
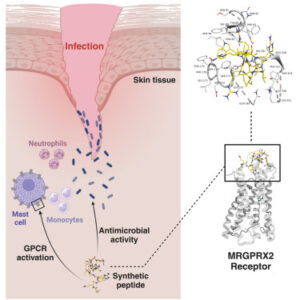
- Membrane Puncture: They physically pierce bacterial cell membranes, leading to rapid eradication of pathogens that often evade traditional antibiotics.
- Immune Activation: They activate the G protein–coupled receptor MRGPRX2 on mast cells—a receptor that was previously considered inactive in this context—resulting in the release of antimicrobial mediators that bolster infection control.
- Enhanced Stability: Their mirror-image D amino–acid configuration makes them highly resistant to protease enzymes, overcoming a major hurdle in peptide therapeutics and paving the way for clinical application.
Harnessing the Body’s Natural Defenses
By targeting MRGPRX2, immune responses are activated in conjunction with conventional antimicrobial approaches. Stimulating MRGPRX2 can boost immune cell activity, producing a synergistic effect—delivering a powerful “one-two punch” against infections that exceeds the effectiveness of traditional antibiotics alone. As antibiotic resistance increases, therapies that combine bacterial eradication with immune system stimulation emerge as a crucial and innovative frontier in the fight against infectious diseases.
Conclusion
The ongoing preclinical studies aim to validate the efficacy of these innovative peptides in animal infection models and to refine dosing strategies, while efforts to develop derivatives targeting other challenging pathogens and immune pathways continue. The mirror-image design of these peptide-based drugs effectively addresses longstanding issues of stability and degradation, paving the way for new anti-infective therapies. If successful, this approach holds great potential to improve protections for vulnerable hospital patients and to combat the global surge of antibiotic-resistant infections. Ultimately, this research represents a promising advancement toward more durable, immune-boosting antimicrobial treatments that could transform the future of infectious disease management.
For more information on this study, please refer to the full paper published in Cell Biomaterials: https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00079-0
For more information, please contact:
Machine Biology Group
University of Pennsylvania
Authors:
Aetas Amponnawarat, Marcelo D.T. Torres, Rakesh Krishnan, Cesar de la Fuente-Nunez, Hydar Ali.
Published:
May 27, 2025
About Machine Biology Group:
The mission statement of the Machine Biology Group at the University of Pennsylvania is to use the power of machines to accelerate discoveries in biology and medicine.
